
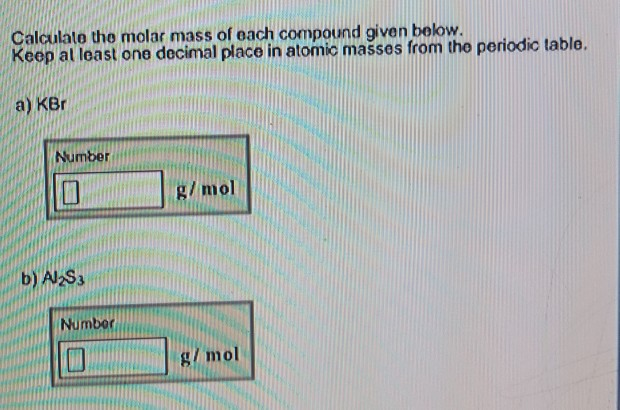
And that is correct, the mass shown in the periodic table is actually the mass of 6.022 x 10 23 carbon atoms or a mole of carbon atoms. This means that 1 AMU ≈ 1g right? and thus Carbon has a mass of 12amu, correct? But wait, this could not be the mass of a single carbon atom right? They are really, really tiny. Let's start the relationship discussion with the relationship between the mole and the AMU.Īn AMU is 1/12 the mass of a Carbon-12 atom which according to the periodic table weighs ~12g. Reactions are balanced based on the number of moles of each element in the reaction, solution concentrations are very often described in terms of moles per liter or moles per kg of solvent and we have already seen that the molecules or atoms of an element are reported as moles of the substance rather than the individual count of their particles. The reason the mole is so important is because we use the mole as the unit for most of the relationships in chemistry. So if we had exactly 18.014g of water we would have 1 mole of water. If we total up the gram amounts of each element in the water molecule = 15.998g/mol + 2(1.008g/mol) we get the molar mass of water = 18.014g/mol. The mass of oxygen equal to one mole of oxygen is 15.998 grams and the mass of one mole of hydrogen is 1.008 g. One mole of Water is composed of 1 mole of Oxygen and two moles of Hydrogen. To use our old friend water as an example: The Molar mass or Molecular Weight (interchangeable terms so long as we are on Earth) of a substance is the total of all the individual masses of the elements it contains.

Moles of a Substance and the Molecular Weight We use the stoichiometry (fancy way of saying mole ratios in an equation) to make predictions about how much product will be made or reactant needed if we know one mole amount in a reaction. What can we do with moles? We use the unit to make calculations based on balanced chemical equations. This is why we state the atomic and molecular masses in units of grams per mole or g/mol. Lithium for instance has an atomic mass of 6.941 grams and this is equal to one mole of lithium. Using carbon as a reference, the atomic weights you see in the periodic table are also equal to one mole of those substances: This means that the atomic mass or atomic weight (12 grams) of carbon is equal to exactly 1 mole of carbon. Why use 12 grams? This is the theoretical atomic mass of the Carbon-12 isotope (6 protons and 6 neutrons). The value given 6.022 x 10 23 is called Avagadro's number for the scientist that found the number of atoms in 12 grams of carbon 12. We use the mole (mol) to represent the amount of substances in chemistry because the numbers of atoms and molecules in each substance is so large. So if you had a mole of donuts you would have 6.022 x 10 23 donuts and a serious stomach ache. So the mole is the title used for the amount 6.022 x 10 23 much the same way the word "dozen" is used for the amount 12. The MOLE (mol) is a unit of measurement that is the amount of a pure substance containing the same number of chemical units (atoms, molecules etc.) as there are atoms in exactly 12 grams of carbon-12 (i.e., 6.022 X 10 23). In this lecture we cover the Mole and Avagadro's Number as well as the calculations for Molar Mass and conversions using moles. The content that follows is the substance of lecture 8.


 0 kommentar(er)
0 kommentar(er)
